Former I-Corps Participant, Popa lands funding to study the ‘orgami’ of life – how proteins fold
By: Laura Otto
Now that the human genome has been mapped, we know more about the role of genes in the body. But proteins are the actual workhorses of genetic instruction, carrying out every function needed for life. So, it makes sense that protein processes also hold valuable information about disease.
UWM biophysicist Ionel Popa is investigating how proteins carry out their essential work – a shape-changing process called “protein folding.” A better understanding of protein folding holds insight into some diseases, which begin when a protein misfolds, he says.
Popa, who joined the UWM physics faculty in 2015, was recently recognized with a Shaw Scientist Award from the Greater Milwaukee Foundation. The 30-year-old program has supported early-career researchers from UWM or UW-Madison working in biochemistry, biological sciences and cancer research.
He is using the $200,000 grant to investigate how proteins assemble and change while folding and unfolding at the cellular level in order to accomplish an enormous number of bodily functions. In particular, he wants to know how mechanical forces at specific sites inside the body affect proteins as they fold or unfold.
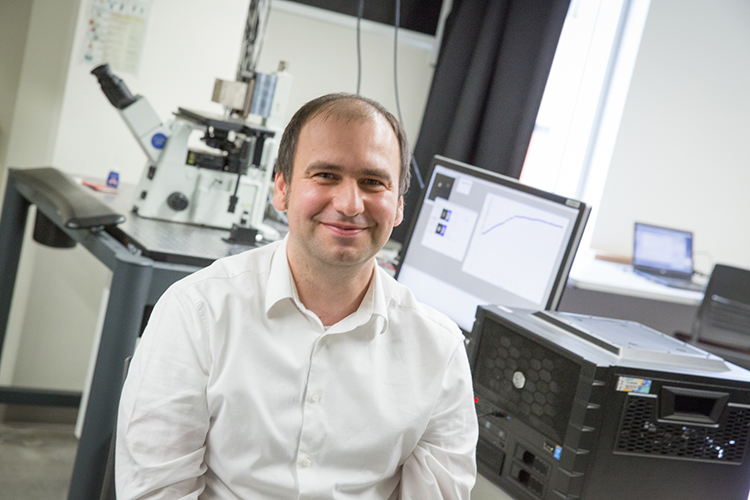
UWM Associate Professor Ionel Popa is testing how mechanical forces affect proteins as they fold and unfold inside the body. Studying this shape-changing process could provide insights into what goes awry and causes disease. (UWM Photo/Troye Fox)
What is protein folding and how much do we know about it?
Proteins are worker bees of the cells. A single protein makes a chain made from 20 different types of amino acids, as encoded by the genes. The protein then needs to fold into three-dimensional shape in order to execute its function. This process is called protein folding.
Protein folding or unfolding can involve mechanical forces that are specific to the particular cell’s environment. The interplay of these forces acts as mechanical signaling to nearby cells, tissues and organs. It’s one of the most complex self-organization processes in nature.
Help us visualize a protein at work.
Think of a protein as multiple knots on a rope. When they are “folded,” all the knots are tight and the rope is stiff. In their unfolded state, the rope is relaxed and the knots are loosened. Applying force to the unfolded state exposes hidden parts that can now interact with the environment.
By increasing the amount of force, you expose more sites that can form more linkages. A protein domain is 4 to 5 nanometers, but can expand to between 10 and 50 nanometers, depending on the force applied. It’s like construction of a building. You connect to what’s there, reinforcing as you add floors.
You can’t see proteins. So how do you study the effect of such forces?
We’ve built our own instrumentation, called “single molecule magnetic tweezers,” to explore protein folding. Using magnetic tweezers, we can make a synthetic protein and then apply forces in a lab setting to test a particular physical process by tethering it between a glass surface and a para-magnetic bead.
Since we can’t see the molecule changing during the test, we measure the position of a para-magnetic bead that is attached at one end, and that of a non-magnetic bead, glued to the surface. Then we apply a magnetic force and measure the unfolding and refolding of each protein.
A profound new concept that is emerging from our experiments with magnetic tweezers is that proteins under force can reverse their domains from folded to unfolded states in vivo (in the body). This continuous unfolding and refolding might have biological implications, such as signaling, storage of energy, or recruitment and release of binding partners.
What can protein folding tell us about disease?
Misfolding is when the protein tries to obtain its final 3-D structure but ends up with a different, toxic structure. Misfolding causes a chain reaction that can lead to disease. But it is a relatively rare occurrence in the body. In our lab, we can speed up the folding process and measure in one hour the same number of times misfolding happens over the span of 60 years.
Give us a specific example of how your work can inform disease.
Using magnetic tweezers, we have studied the mechanical response of titin, the giant protein that gives muscles their elasticity. In a recent article, we proposed a new mechanism for muscle contraction: As muscles contract and relax, the unfolding and refolding of some of titin’s domains play an active role.
Understanding how certain mutations change the working range of proteins that drive contraction will help us investigate muscular dystrophy, which manifests as an increasing weakening and breakdown of skeletal muscles over time.
Read the original article here.